Silicone hose type NeoSil 1000B-HP
The translucent silicone hose type NeoSil 1000B-HP owes its name to its high-purity design. The entire silicone hose* is manufactured in an Class 8 clean room, according to ISO 14644 ensuring it meets the highest standards of cleanliness, hygiene and ease of cleaning. Between the hose liner and the cover there are fabric inlays combined with a stainless steel helix, which give the silicone hose the required stability and pressure resistance. In addition, the platinum cured liner ensures optimal chemical resistance. The silicone hose with stainless steel helix is resistant to heat, abrasion, ozone and weathering. During production, the hose liner is seamlessly extruded using a special process, which allows longer hoses featuring a particularly smooth inner surface to be produced. This means that our platinum cured silicone hose with stainless steel helix is also available in longer lengths. Due to its premium composition, the silicone hose with stainless steel helix is used in the pharmaceutical, cosmetics and food industries to connect pipelines, plant components and reactors. The silicone hose type NeoSil 1000B HP is completely traceable by reference to the lot number on the cover and, in the case of prefabricated pipelines, by reference to the lot number and an additional serial number on the crimp collar. *The hose consists of the liner, fabric inlays, wire helix and cover (without fittings).
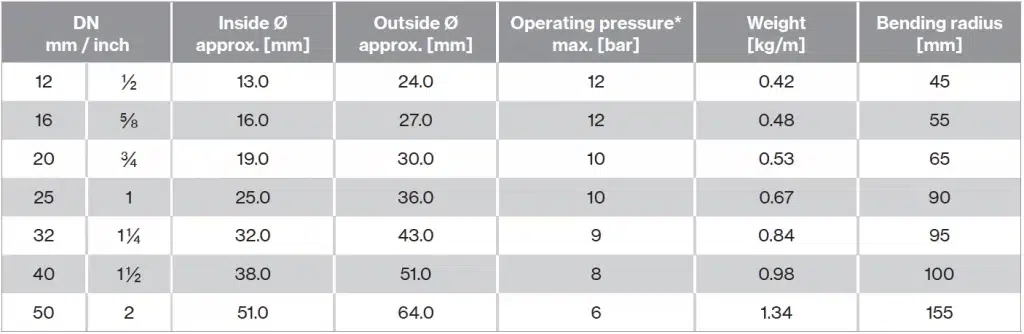
Technical specifications
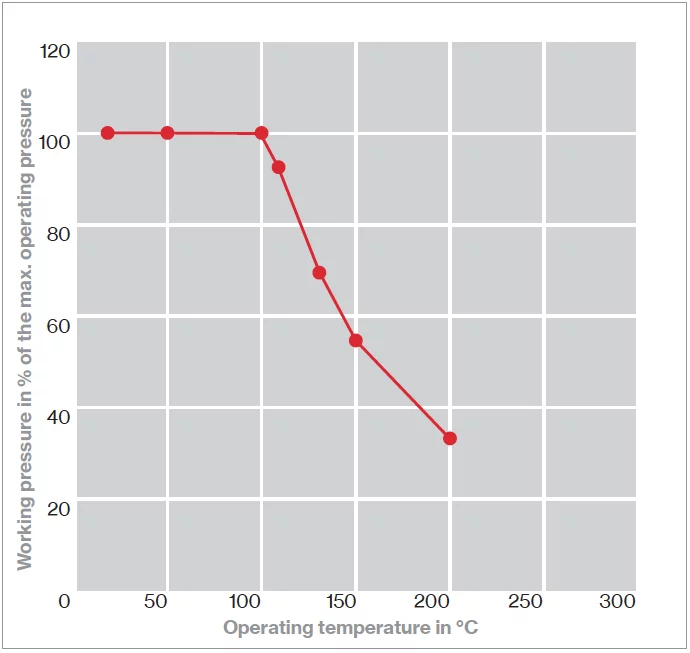
p-T diagram
Structure
| Core | Silicone, platinum cured |
| Cover | Silicone, platinum cured |
| Braiding | N/A |
| Fittings | Crimped |
| Inserts | Fabric inlay, stainless steel helix |
| Temperature | -60 °C / +200 °C |
| Vacuum | At 20 °C: 200 mbar absolute |
| Max. length | 40 m |
| Standard/approval | FDA CFR 21 PART 177.2600, USP XXXVI class VI, European Regulation 1935/2004/CE, European Pharmacopoeia 3.1.9 Ed. VIII / 2014, BfR Recommendation XV, ISO 10993 – 4:2017, 5:2009, 12:2012, 3A Sanitary Standard Class I, Arrêté du 25 novembre 1992, Japan Ministry of Health and Welfare Notice No. 370, 1959, No. 201, 2006 and revision 2012 |